how many valence electrons does s have|Valence electrons (video) : Baguio Mar 23, 2023 Update 3P animation names translation.txt by @IDontHaveIdea in #134; Fix some missing quotes by @ManlyMarco in #137; merge latest translations from KK by @GeBo1 in #138; . Koikatsu Sunshine Translations v1 - 37.830% complete. 30 Sep 10:23 . ManlyMarco. v1 d87cb53.
PH0 · Valence electrons (video)
PH1 · Valence electron
PH2 · Valence Electrons Chart for All Elements
PH3 · Valence Electrons
PH4 · How to Find the Valence Electrons for Sulfur (S)?
PH5 · How many valence electrons does S have?
PH6 · How Many Valence Electrons Does Sulfur (S) Have?
PH7 · Determine valence electrons using the periodic table
PH8 · 3.1: Valence Electrons
PH9 · 3.10: Valence Electrons
PH10 · 10.6: Valence Electrons
Time Zone Converter – Time Difference Calculator. Provides time zone conversions taking into account Daylight Saving Time (DST), local time zone and accepts present, past, or future dates.
how many valence electrons does s have*******Mar 23, 2023 Learn how to determine the number of valence electrons for an element using the periodic table. See the pattern for main group elements and the exceptions for h. The element of group 16 is sulfur and its symbol is ‘S’. The valence electrons are the total number of electrons in the last orbit (shell). The total number of electrons in the last shell after the electron .
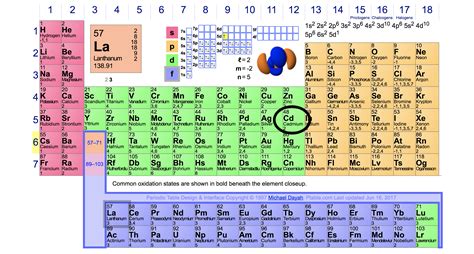
Valence electrons are the electrons in the outermost shell, or energy level, of an atom. For example, oxygen has six valence electrons, two in the 2s subshell and four in the 2p . Example \(\PageIndex{1}\): Number of Valence Electrons. How many valence electrons are in one atom of each element? sulfur; helium; potassium; .
Carbon has four valence electrons and here a valence of four. Each hydrogen atom has one valence electron and is univalent. In chemistry and physics, valence electrons are .

Valence electrons are the electrons in the highest occupied principal energy level of an atom. In the second period elements, the two electrons in the 1s 1 s sublevel are called .
Valence electrons (video) Learn how valence electrons determine chemical properties and bonding of elements. Find out how many valence electrons each element has by looking at its Group in the periodic .how many valence electrons does s have How many valence electrons does boron have? Recognize that the second principal energy level consists of both the \(2s\) and the \(2p\) sublevels, and so the .
how many valence electrons does s have Valence electrons (video) How many valence electrons does boron have? Recognize that the second principal energy level consists of both the \(2s\) and the \(2p\) sublevels, and so the .
Valence electrons are the electrons present in the outermost shell of an atom. You can easily determine the number of valence electrons an atom can have by looking at its Group in the periodic table. For example, atoms in Groups 1 and 2 have 1 and 2 valence electrons, respectively. Atoms in Groups 13 and 18 have 3 and 8 valence electrons . 6 valence electrons. Explanation: Sulfur is in the sixteenth column of the periodic table, and it has a 2- charge. Therefore it will have 8 − 2 electrons in the outer, or valence shell. ∴ Sulfur has 6 valence electrons. All the members of the same family (column) on the periodic table will have the same number of valence electrons. Solution. Element A is located in Period 2, the 5th position in 2p-block.Before the electrons are placed in 2p subshell, the 2s subshell must be filled first. This means that A has two valence electrons in 2s (2s 2) and five valence electrons in 2p (2p 5).Answer: 2s 2 2p 5. It has 2 + 5 = 7 valence electrons.. Element B is located in Period 3, the 2nd .
Beryllium has two valence electrons. How many valence electrons does boron have? Recognize that the second principal energy level consists of both the \(2s\) and the \(2p\) sublevels, and so the answer is three. In fact, the number of valence electrons goes up by one for each step across a period, until the last element is .
How many valence electrons does boron have? Recognize that the second principal energy level consists of both the \(2s\) and the \(2p\) sublevels, and so the answer is three. B: 1s 2 2s 2 2p 1 (there are three electrons on the highest occupied energy level n=2) In fact, the number of valence electrons goes up by one for each step across a .
We know that arsenic atoms have a total of thirty-three electrons. The electron configuration shows that the first shell of arsenic has two electrons, the second shell has eight electrons, the 3rd shell has eighteen electrons and the 4th shell has five electrons. Therefore, the number of electrons per shell of arsenic is 2, 8, 18, 5. This table of element valences includes the maximum valence and most common valence values in chemistry. Use this for reference with a periodic table. . so you can't always predict an element's behavior just by counting these outer electrons. Nevertheless, it's useful to know the most common valences. Here is a table of element .Every subshell has a # of orbits s/p/d/f that can each hold 2 electrons each (one has the opposite spin of the other). The first shell (of all atoms) has 1 subshell of s-orbitals containing 1 s orbital. This means that the first shell can hold 2 electrons. The second shell has 2 subshells: 1 s-orbital and 3 p-orbitals.
When forming ions, elements typically gain or lose the minimum number of electrons necessary to achieve a full octet. For example, fluorine has seven valence electrons, so it is most likely to gain one electron to form an ion with a 1- charge. We can use this method to predict the charges of ions in ionic compounds.How many valence electrons does boron have? You must recognize that the second principal energy level consists of both the \(2s\) and the \(2p\) sublevels and so the answer is three. In fact, the number of valence electrons goes up by one for each step across a period until the last element is reached. Neon, with its configuration ending in .The valence (or valency) of an element is a measure of its combining power with other atoms when it forms chemical compounds or molecules. The concept of valence was developed in the last half of the 19th century and was successful in explaining the molecular structure of many organic compounds. The quest for the underlying causes of valence .
Solution. Steps for Writing Lewis Structures. Example 15.4.1 15.4. 1. 1. Determine the total number of valence electrons in the molecule or ion. Each H atom (group 1) has 1 valence electron, and the O atom (group 16) has 6 valence electrons, for a total of 8 valence electrons. 2. Sulfur is a classified nonmetal element and its symbol is S. Sulfur is the 16th element of the periodic table so its atomic number is 16. The atomic number of an element is equal to the number of protons and . Answer: Aluminum has 3 valence electrons. Explanation: Valence electrons are the electrons present in the outermost energy level (shell) of an atom. Aluminum (Al), with an atomic number of 13, has an electron configuration of 1s 2 2s 2 2p 6 3s 2 3p 1. In this configuration, the outermost shell is the third shell (3s 2 3p 1 ), which .
The valence electrons are the electrons that determine the most typical bonding patterns for an element. These electrons are found in the s and p orbitals of the highest energy level for the element. Sodium 1s22s22p63s1. Sodium has 1 valence electron from the 3s orbital. Phosphorus 1s22s22p63s23p3. Phosphorus has 5 valence electrons 2 from the . In the case of titanium, it has an atomic number of 22, which means it has 22 electrons. The electronic configuration of titanium is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 2. The valence electrons of an atom are the electrons in the outermost energy level or shell.
So that means that sodium has one valence electron. And that's very convenient, because sodium is found in group one. And so we can say that for main groups, if you want to figure out how many valence electrons you have, it's just equal to the group number. So the group number is equal to the number of valence electrons. Chlorine has 7 valence electrons . > The electron configuration of chlorine is 1s^2 2s^2 2p^6 3s^2 3p^5 or "[Ne]"3s^2 3p^5. The 3s^2 3p^5 electrons are the outermost electrons, so chlorine has seven valence electrons. In a picture, the valence electrons are the ones in the outermost shell. You can see in the diagram below that .
The SEO Penrith team that makes a positive difference in your online marketing. A site That Works For You is a team of web professionals that includes developers, SEO gurus, content creators and project managers. We have guided and helped people along the often uneven landscape of search engine optimisation. And, we’ve achieved successes .
how many valence electrons does s have|Valence electrons (video)